Why Rural Healthcare Needs a 'Make in India' Campaign
- BY Ira Swasti
 In Technology
In Technology 9487
9487 0
0
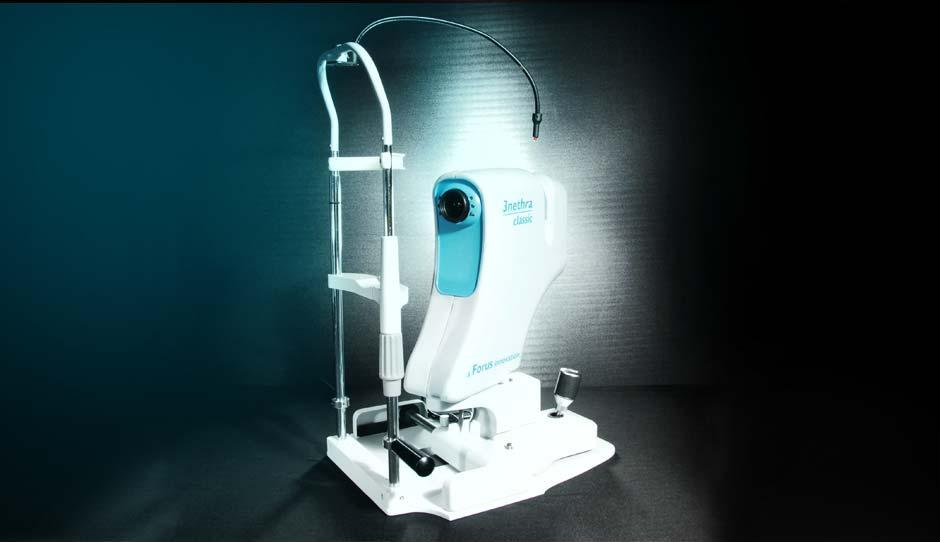
The ophthalmologist to patient ratio in India is so skewed at 1:70000 that we knew we could only solve the problem through technology which was scalable." -Dr. K Chandrashekhar
According to Dr Ruchi Dass, CEO & founder, HealthCursor Consulting Group, 80 per cent of India’s population lives in semi-urban or rural areas, yet more than 70 per cent health facilities are available in urban areas. And, if we look at the medical devices market specifically, 65 per cent of all medical devices are imported from outside India, even when the cheap medical device market in India is worth $5.2 billion. Clearly, this is a hugely untapped opportunity in the market.

But thankfully, there are companies such as Forus, Skanray and Perfint Healthcare that are heralding a new wave of medical innovation in India with their indigenously developed medical devices. It was in 2005 that two colleagues at Phillips—Dr Shyam Vasudeva Rao and K. Chandrasekhar heard a lecture by Dr Arvind of the Arvind Eye Care hospital chain and learnt that 80 per cent of blindness cases in India were preventable. Ready to take on the challenge, the co-workers took four years of research and analysis, quit their jobs in 2009, and built 3nethra, an integrated eye screening device which combines three other individual components into one, to detect major eye ailments such as cataract, glaucoma, diabetic retina and cornea problems in a fast and accurate manner. “The ophthalmologist to patient ratio in India is so skewed at 1:70000 that we knew we could only solve this problem through technology which was scalable,” says Chandrasekhar, co-founder, Forus Healthcare.
Priced at Rs5 to Rs7 lakh, 3nethra not only brings down the cost of eye screening by four times as compared to the three individual devices required to do the same level of eye screening but it is portable (at about 13kg) and can be used by paramedical staff with minimal training, as is usually the case in villages or urban slums, where medical infrastructure and trained personnel are hard to find. “The main idea of building this device was to democratise the process of eye screening. So we first targeted hospitals, then eye care specialists, then optometricians and now optometric shops, to install our devices,” says Chandrasekhar.

With over 130 installations, today, the device reaches 3,50,000 to 4,00,00 people in 15 states. Out of these, about 60per cent of the installations are in metros and Tier 1 cities while 40 per cent are in non metros. Though, the company’s focus while building 3nethra was not so much on segmenting the market but making the product usable in different scenarios— whether it was a vision centre, a mobile van or an OPD. “In fact, many of our devices installed in urban Bangalore are taken to nearby villages by medical staff to cater to the rural population on weekends,” he says. But making a medical device in India is not without its own set of challenges because of the lack of an ecosystem. There are not enough medical devices companies in India nor enough human resources with the required skills. Plus, validation of a health care product is more complex and requires more time than a consumer product.
Finding investors to fund product development amounts to another challenge as investors usually wish to see a prototype before they invest in the business, which creates a chicken and egg problem as entrepreneurs find themselves with no funds to conduct research and build a prototype. Luckily for Forus though, Accel Partners and IDG Ventures provided angel funding of $5 million, which along with the founders own money, was enough to build the product and launch in the market. The two-year-old firm today has more ambitious plans for its future. It hopes to expand its markets outside India, starting with Africa and Latin America in the next two years. “Thanks to the universal design of the product, 3nethra can be used in both urban and rural settings, anywhere in the world.”




























Add new comment